Photodiode
From Wikipedia, the free encyclopedia
(Redirected from Phototransistor)
A photodiode is a type of photodetector capable of converting light into either current or voltage, depending upon the mode of operation.[1]Photodiodes are similar to regular semiconductor diodes except that they may be either exposed (to detect vacuum UV or X-rays) or packaged with a window or optical fiber connection to allow light to reach the sensitive part of the device. Many diodes designed for use specifically as a photodiode will also use a PIN junction rather than the typical PN junction.
Contents[hide] |
[edit] Principle of operation
A photodiode is a PN junction or PIN structure. When a photon of sufficient energy strikes the diode, it excites an electron, thereby creating a free electron and a (positively charged electron) hole. If the absorption occurs in the junction's depletion region, or one diffusion length away from it, these carriers are swept from the junction by the built-in field of the depletion region. Thus holes move toward the anode, and electrons toward the cathode, and a photocurrent is produced.[edit] Photovoltaic mode
When used in zero bias or photovoltaic mode, the flow of photocurrent out of the device is restricted and a voltage builds up. The diode becomes forward biased and "dark current" begins to flow across the junction in the direction opposite to the photocurrent. This mode is responsible for the photovoltaic effect, which is the basis for solar cells – in fact, a solar cell is just a large area photodiode.[edit] Photoconductive mode
In this mode the diode is often reverse biased, dramatically reducing the response time at the expense of increased noise. This increases the width of the depletion layer, which decreases the junction's capacitance resulting in faster response times. The reverse bias induces only a small amount of current (known as saturation or back current) along its direction while the photocurrent remains virtually the same. The photocurrent is linearly proportional to the illuminance.[1]Although this mode is faster, the photoconductive mode tends to exhibit more electronic noise.[citation needed] The leakage current of a good PIN diode is so low (< 1nA) that the Johnson–Nyquist noise of the load resistance in a typical circuit often dominates.
[edit] Other modes of operation
Avalanche photodiodes have a similar structure to regular photodiodes, but they are operated with much higher reverse bias. This allows each photo-generated carrier to be multiplied by avalanche breakdown, resulting in internal gain within the photodiode, which increases the effective responsivity of the device.Phototransistors also consist of a photodiode with internal gain. A phototransistor is in essence nothing more than a bipolar transistor that is encased in a transparent case so that light can reach the base-collector junction. The electrons that are generated by photons in the base-collector junction are injected into the base, and this photodiode current is amplified by the transistor's current gain β (or hfe). Note that while phototransistors have a higher responsivity for light they are not able to detect low levels of light any better than photodiodes.[citation needed] Phototransistors also have significantly longer response times.
[edit] Materials
The material used to make a photodiode is critical to defining its properties, because only photons with sufficient energy to excite electrons across the material's bandgap will produce significant photocurrents.Materials commonly used to produce photodiodes include[2]:
| Material | Electromagnetic spectrum wavelength range (nm) |
|---|---|
| Silicon | 190 – 1100 |
| Germanium | 400 – 1700 |
| Indium gallium arsenide | 800 – 2600 |
| Lead(II) sulfide | <1000 – 3500 |
[edit] Unwanted photodiodes
Since transistors and ICs are made of semiconductors, and contain P-N junctions, almost every active component is potentially a photodiode. Many components, especially those sensitive to small currents, will not work correctly if illuminated, due to the induced photocurrents. In most components this is not desired, so they are placed in an opaque housing. Since housings are not completely opaque to X-rays or other high energy radiation, these can still cause many ICs to malfunction due to induced photo-currents.[edit] Features
Critical performance parameters of a photodiode include:- Responsivity
- The ratio of generated photocurrent to incident light power, typically expressed in A/W when used in photoconductive mode. The responsivity may also be expressed as a Quantum efficiency, or the ratio of the number of photogenerated carriers to incident photons and thus a unitless quantity.
- Dark current
- The current through the photodiode in the absence of light, when it is operated in photoconductive mode. The dark current includes photocurrent generated by background radiation and the saturation current of the semiconductor junction. Dark current must be accounted for by calibration if a photodiode is used to make an accurate optical power measurement, and it is also a source of noise when a photodiode is used in an optical communication system.
- Noise-equivalent power
- (NEP) The minimum input optical power to generate photocurrent, equal to the rms noise current in a 1 hertz bandwidth. The related characteristic detectivity (D) is the inverse of NEP, 1/NEP; and the specific detectivity (
 ) is the detectivity normalized to the area (A) of the photodetector,
) is the detectivity normalized to the area (A) of the photodetector,  . The NEP is roughly the minimum detectable input power of a photodiode.
. The NEP is roughly the minimum detectable input power of a photodiode.
[edit] Applications
P-N photodiodes are used in similar applications to other photodetectors, such as photoconductors, charge-coupled devices, and photomultiplier tubes.Photodiodes are used in consumer electronics devices such as compact disc players, smoke detectors, and the receivers for remote controls in VCRs and televisions.
In other consumer items such as camera light meters, clock radios (the ones that dim the display when it's dark) and street lights, photoconductors are often used rather than photodiodes, although in principle either could be used.
Photodiodes are often used for accurate measurement of light intensity in science and industry. They generally have a better, more linear response than photoconductors.
They are also widely used in various medical applications, such as detectors for computed tomography (coupled with scintillators) or instruments to analyze samples (immunoassay). They are also used in pulse oximeters.
PIN diodes are much faster and more sensitive than ordinary p-n junction diodes, and hence are often used for optical communications and in lighting regulation.
P-N photodiodes are not used to measure extremely low light intensities. Instead, if high sensitivity is needed, avalanche photodiodes, intensified charge-coupled devices or photomultiplier tubes are used for applications such as astronomy, spectroscopy, night vision equipment and laser rangefinding.
[edit] Comparison with photomultipliers
Advantages compared to photomultipliers:- Excellent linearity of output current as a function of incident light
- Spectral response from 190 nm to 1100 nm (silicon), longer wavelengths with other semiconductor materials
- Low noise
- Ruggedized to mechanical stress
- Low cost
- Compact and light weight
- Long lifetime
- High quantum efficiency, typically 80%
- No high voltage required
- Small area
- No internal gain (except avalanche photodiodes, but their gain is typically 102–103 compared to up to 108 for the photomultiplier)
- Much lower overall sensitivity
- Photon counting only possible with specially designed, usually cooled photodiodes, with special electronic circuits
- Response time for many designs is slower
[edit] P-N vs. P-I-N photodiodes
- Due to the intrinsic layer, a PIN photodiode must be reverse biased (Vr). The Vr increases the depletion region allowing a larger volume for electron-hole pair production, and reduces the capacitance thereby increasing the bandwidth.
- The Vr also introduces noise current, which reduces the S/N ratio. Therefore, a reverse bias is recommended for higher bandwidth applications and/or applications where a wide dynamic range is required.
- A PN photodiode is more suitable for lower light applications because it allows for unbiased operation.
[edit] Photodiode array
Hundreds or thousands (up to 2048) photodiodes of typical sensitive area 0.025 x 1 mm each arranged as a one-dimensional array, which can be used as a position sensor. One advantage of photodiode arrays (PDAs) is that they allow for high speed parallel read out since the driving electronics may not be built in like a traditional CMOS or CCD sensor.Laser diode
From Wikipedia, the free encyclopedia

A packaged laser diode with penny for scale.
Contents[hide] |
[edit] Theory of operation
A laser diode, like many other semiconductor devices, is formed by doping a very thin layer on the surface of a crystal wafer. The crystal is doped to produce an n-type region and a p-type region, one above the other, resulting in a p-n junction, or diode.Laser diodes form a subset of the larger classification of semiconductor p-n junction diodes. As with any semiconductor p-n junction diode, forward electrical bias causes the two species of charge carrier – holes and electrons – to be "injected" from opposite sides of the p-n junction into the depletion region, situated at its heart. Holes are injected from the p-doped, and electrons from the n-doped, semiconductor. (A depletion region, devoid of any charge carriers, forms automatically and unavoidably as a result of the difference in chemical potential between n- and p-type semiconductors wherever they are in physical contact.)
As charge injection is a distinguishing feature of diode lasers as compared to all other lasers, diode lasers are traditionally and more formally called "injection lasers." (This terminology differentiates diode lasers, e.g., from flashlamp-pumped solid state lasers, such as the ruby laser. Interestingly, whereas the term "solid-state" was extremely apt in differentiating 1950s-era semiconductor electronics from earlier generations of vacuum electronics, it would not have been adequate to convey unambiguously the unique characteristics defining 1960s-era semiconductor lasers.) When an electron and a hole are present in the same region, they may recombine or "annihilate" with the result being spontaneous emission — i.e., the electron may re-occupy the energy state of the hole, emitting a photon with energy equal to the difference between the electron and hole states involved. (In a conventional semiconductor junction diode, the energy released from the recombination of electrons and holes is carried away as phonons, i.e., lattice vibrations, rather than as photons.) Spontaneous emission gives the laser diode below lasing threshold similar properties to an LED. Spontaneous emission is necessary to initiate laser oscillation, but it is one among several sources of inefficiency once the laser is oscillating.
The difference between the photon-emitting semiconductor laser and conventional phonon-emitting (non-light-emitting) semiconductor junction diodes lies in the use of a different type of semiconductor, one whose physical and atomic structure confers the possibility for photon emission. These photon-emitting semiconductors are the so-called "direct bandgap" semiconductors. The properties of silicon and germanium, which are single-element semiconductors, have bandgaps that do not align in the way needed to allow photon emission and are not considered "direct." Other materials, the so-called compound semiconductors, have virtually identical crystalline structures as silicon or germanium but use alternating arrangements of two different atomic species in a checkerboard-like pattern to break the symmetry. The transition between the materials in the alternating pattern creates the critical "direct bandgap" property. Gallium arsenide, indium phosphide, gallium antimonide, and gallium nitride are all examples of compound semiconductor materials that can be used to create junction diodes that emit light.
In the absence of stimulated emission (e.g., lasing) conditions, electrons and holes may coexist in proximity to one another, without recombining, for a certain time, termed the "upper-state lifetime" or "recombination time" (about a nanosecond for typical diode laser materials), before they recombine. Then a nearby photon with energy equal to the recombination energy can cause recombination by stimulated emission. This generates another photon of the same frequency, travelling in the same direction, with the same polarization and phase as the first photon. This means that stimulated emission causes gain in an optical wave (of the correct wavelength) in the injection region, and the gain increases as the number of electrons and holes injected across the junction increases. The spontaneous and stimulated emission processes are vastly more efficient in direct bandgap semiconductors than in indirect bandgap semiconductors; therefore silicon is not a common material for laser diodes.
As in other lasers, the gain region is surrounded with an optical cavity to form a laser. In the simplest form of laser diode, an optical waveguide is made on that crystal surface, such that the light is confined to a relatively narrow line. The two ends of the crystal are cleaved to form perfectly smooth, parallel edges, forming a Fabry–Pérot resonator. Photons emitted into a mode of the waveguide will travel along the waveguide and be reflected several times from each end face before they are emitted. As a light wave passes through the cavity, it is amplified by stimulated emission, but light is also lost due to absorption and by incomplete reflection from the end facets. Finally, if there is more amplification than loss, the diode begins to "lase".
Some important properties of laser diodes are determined by the geometry of the optical cavity. Generally, in the vertical direction, the light is contained in a very thin layer, and the structure supports only a single optical mode in the direction perpendicular to the layers. In the lateral direction, if the waveguide is wide compared to the wavelength of light, then the waveguide can support multiple lateral optical modes, and the laser is known as "multi-mode". These laterally multi-mode lasers are adequate in cases where one needs a very large amount of power, but not a small diffraction-limited beam; for example in printing, activating chemicals, or pumping other types of lasers.
In applications where a small focused beam is needed, the waveguide must be made narrow, on the order of the optical wavelength. This way, only a single lateral mode is supported and one ends up with a diffraction-limited beam. Such single spatial mode devices are used for optical storage, laser pointers, and fiber optics. Note that these lasers may still support multiple longitudinal modes, and thus can lase at multiple wavelengths simultaneously.
The wavelength emitted is a function of the band-gap of the semiconductor and the modes of the optical cavity. In general, the maximum gain will occur for photons with energy slightly above the band-gap energy, and the modes nearest the gain peak will lase most strongly. If the diode is driven strongly enough, additional side modes may also lase. Some laser diodes, such as most visible lasers, operate at a single wavelength, but that wavelength is unstable and changes due to fluctuations in current or temperature.
Due to diffraction, the beam diverges (expands) rapidly after leaving the chip, typically at 30 degrees vertically by 10 degrees laterally. A lens must be used in order to form a collimated beam like that produced by a laser pointer. If a circular beam is required, cylindrical lenses and other optics are used. For single spatial mode lasers, using symmetrical lenses, the collimated beam ends up being elliptical in shape, due to the difference in the vertical and lateral divergences. This is easily observable with a red laser pointer.
The simple diode described above has been heavily modified in recent years to accommodate modern technology, resulting in a variety of types of laser diodes, as described below.
[edit] Types
The simple laser diode structure, described above, is extremely inefficient. Such devices require so much power that they can only achieve pulsed operation without damage. Although historically important and easy to explain, such devices are not practical.[edit] Double heterostructure lasers
In these devices, a layer of low bandgap material is sandwiched between two high bandgap layers. One commonly-used pair of materials is gallium arsenide (GaAs) with aluminium gallium arsenide (AlxGa(1-x)As). Each of the junctions between different bandgap materials is called a heterostructure, hence the name "double heterostructure laser" or DH laser. The kind of laser diode described in the first part of the article may be referred to as a homojunction laser, for contrast with these more popular devices.The advantage of a DH laser is that the region where free electrons and holes exist simultaneously—the active region—is confined to the thin middle layer. This means that many more of the electron-hole pairs can contribute to amplification—not so many are left out in the poorly amplifying periphery. In addition, light is reflected from the heterojunction; hence, the light is confined to the region where the amplification takes place.
[edit] Quantum well lasers
Main article: Quantum well laser
If the middle layer is made thin enough, it acts as a quantum well. This means that the vertical variation of the electron's wavefunction, and thus a component of its energy, is quantized. The efficiency of a quantum well laser is greater than that of a bulk laser because the density of states function of electrons in the quantum well system has an abrupt edge that concentrates electrons in energy states that contribute to laser action.Lasers containing more than one quantum well layer are known as multiple quantum well lasers. Multiple quantum wells improve the overlap of the gain region with the optical waveguide mode.
Further improvements in the laser efficiency have also been demonstrated by reducing the quantum well layer to a quantum wire or to a "sea" of quantum dots.
[edit] Quantum cascade lasers
Main article: Quantum cascade laser
In a quantum cascade laser, the difference between quantum well energy levels is used for the laser transition instead of the bandgap. This enables laser action at relatively long wavelengths, which can be tuned simply by altering the thickness of the layer. They are heterojunction lasers.[edit] Separate confinement heterostructure lasers
The problem with the simple quantum well diode described above is that the thin layer is simply too small to effectively confine the light. To compensate, another two layers are added on, outside the first three. These layers have a lower refractive index than the centre layers, and hence confine the light effectively. Such a design is called a separate confinement heterostructure (SCH) laser diode.Almost all commercial laser diodes since the 1990s have been SCH quantum well diodes.
[edit] Distributed feedback lasers
Main article: Distributed feedback laser
Distributed feedback lasers (DFB) are the most common transmitter type in DWDM-systems. To stabilize the lasing wavelength, a diffraction grating is etched close to the p-n junction of the diode. This grating acts like an optical filter, causing a single wavelength to be fed back to the gain region and lase. Since the grating provides the feedback that is required for lasing, reflection from the facets is not required. Thus, at least one facet of a DFB is anti-reflection coated. The DFB laser has a stable wavelength that is set during manufacturing by the pitch of the grating, and can only be tuned slightly with temperature. DFB lasers are widely used in optical communication applications where a precise and stable wavelength is critical.[edit] VCSELs
Main article: Vertical-cavity surface-emitting laser
Vertical-cavity surface-emitting lasers (VCSELs) have the optical cavity axis along the direction of current flow rather than perpendicular to the current flow as in conventional laser diodes. The active region length is very short compared with the lateral dimensions so that the radiation emerges from the surface of the cavity rather than from its edge as shown in the figure. The reflectors at the ends of the cavity are dielectric mirrors made from alternating high and low refractive index quarter-wave thick multilayer.Such dielectric mirrors provide a high degree of wavelength-selective reflectance at the required free surface wavelength λ if the thicknesses of alternating layers d1 and d2 with refractive indices n1 and n2 are such that n1d1 + n2d2 = 1 / 2λ which then leads to the constructive interference of all partially reflected waves at the interfaces. But there is a disadvantage: because of the high mirror reflectivities, VCSELs have lower output powers when compared to edge-emitting lasers.
There are several advantages to producing VCSELs when compared with the production process of edge-emitting lasers. Edge-emitters cannot be tested until the end of the production process. If the edge-emitter does not work, whether due to bad contacts or poor material growth quality, the production time and the processing materials have been wasted. Additionally, because VCSELs emit the beam perpendicular to the active region of the laser as opposed to parallel as with an edge emitter, tens of thousands of VCSELs can be processed simultaneously on a three inch Gallium Arsenide wafer. Furthermore, even though the VCSEL production process is more labor and material intensive, the yield can be controlled to a more predictable outcome.
[edit] VECSELs
Main article: Vertical-external-cavity surface-emitting-laser
Vertical external-cavity surface-emitting lasers, or VECSELs, are similar to VCSELs. In VCSELs, the mirrors are typically grown epitaxially as part of the diode structure, or grown separately and bonded directly to the semiconductor containing the active region. VECSELs are distinguished by a construction in which one of the two mirrors is external to the diode structure. As a result, the cavity includes a free-space region. A typical distance from the diode to the external mirror would be 1 cm.One of the most interesting features of any VECSEL is the small thickness of the semiconductor gain region in the direction of propagation, less than 100 nm. In contrast, a conventional in-plane semiconductor laser entails light propagation over distances of from 250 µm upward to 2 mm or longer. The significance of the short propagation distance is that it causes the effect of "antiguiding" nonlinearities in the diode laser gain region to be minimized. The result is a large-cross-section single-mode optical beam which is not attainable from in-plane ("edge-emitting") diode lasers.
Several workers demonstrated optically pumped VECSELs, and they continue to be developed for many applications including high power sources for use in industrial machining (cutting, punching, etc.) because of their unusually high power and efficiency when pumped by multi-mode diode laser bars.
Electrically pumped VECSELs have also been demonstrated. Applications for electrically pumped VECSELs include projection displays, served by frequency doubling of near-IR VECSEL emitters to produce blue and green light.
[edit] External-cavity diode lasers
External-cavity diode lasers are tunable lasers which use mainly double heterostructures diodes of the AlxGa(1-x)As type. The first external-cavity diode lasers used intracavity etalons[1] and simple tuning Littrow gratings.[2] Other designs include gratings in grazing-incidence configuration and multiple-prism grating configurations.[3][edit] Failure modes
Laser diodes have the same reliability and failure issues as light emitting diodes. In addition they are subject to catastrophic optical damage (COD) when operated at higher power.Many of the advances in reliability of diode lasers in the last 20 years remain proprietary to their developers. The reliability of a laser diode can make or break a product line. Moreover, "reverse engineering" is not always able to reveal the differences between more-reliable and less-reliable diode laser products.
At the edge of a diode laser, where light is emitted, a mirror is traditionally formed by cleaving the semiconductor wafer to form a specularly reflecting plane. This approach is facilitated by the weakness of the [110] crystallographic plane in III-V semiconductor crystals (such as GaAs, InP, GaSb, etc.) compared to other planes. A scratch made at the edge of the wafer and a slight bending force causes a nearly atomically perfect mirror-like cleavage plane to form and propagate in a straight line across the wafer.
But it so happens that the atomic states at the cleavage plane are altered (compared to their bulk properties within the crystal) by the termination of the perfectly periodic lattice at that plane. Surface states at the cleaved plane, have energy levels within the (otherwise forbidden) bandgap of the semiconductor.
Essentially, as a result when light propagates through the cleavage plane and transits to free space from within the semiconductor crystal, a fraction of the light energy is absorbed by the surface states whence it is converted to heat by phonon-electron interactions. This heats the cleaved mirror. In addition the mirror may heat simply because the edge of the diode laser—which is electrically pumped—is in less-than-perfect contact with the mount that provides a path for heat removal. The heating of the mirror causes the bandgap of the semiconductor to shrink in the warmer areas. The bandgap shrinkage brings more electronic band-to-band transitions into alignment with the photon energy causing yet more absorption. This is thermal runaway, a form of positive feedback, and the result can be melting of the facet, known as catastrophic optical damage, or COD.
In the 1970s this problem, which is particularly nettlesome for GaAs-based lasers emitting between 1 µm and 0.630 µm wavelengths (less so for InP based lasers used for long-haul telecommunications which emit between 1.3 µm and 2 µm), was identified. Michael Ettenberg, a researcher and later Vice President at RCA Laboratories' David Sarnoff Research Center in Princeton, New Jersey, devised a solution. A thin layer of aluminum oxide was deposited on the facet. If the aluminum oxide thickness is chosen correctly it functions as an anti-reflective coating, reducing reflection at the surface. This alleviated the heating and COD at the facet.
Since then, various other refinements have been employed. One approach is to create a so-called non-absorbing mirror (NAM) such that the final 10 µm or so before the light emits from the cleaved facet are rendered non-absorbing at the wavelength of interest.
In the very early 1990s, SDL, Inc. began supplying high power diode lasers with good reliability characteristics. CEO Donald Scifres and CTO David Welch presented new reliability performance data at, e.g., SPIE Photonics West conferences of the era. The methods used by SDL to defeat COD were considered to be highly proprietary and have still not been disclosed publicly as of June, 2006.
In the mid-1990s IBM Research (Ruschlikon, Switzerland) announced that it had devised its so-called "E2 process" which conferred extraordinary resistance to COD in GaAs-based lasers. This process, too, has never been disclosed as of June, 2006.
Reliability of high-power diode laser pump bars (employed to pump solid state lasers) remains a difficult problem in a variety of applications, in spite of these proprietary advances. Indeed, the physics of diode laser failure is still being worked out and research on this subject remains active, if proprietary.
Extension of the lifetime of laser diodes is critical to their continued adaptation to a wide variety of applications.
[edit] Applications of laser diodes

Laser diodes can be arrayed to produce very high power (continuous wave or pulsed) outputs. Such arrays may be used to efficiently pump solid state lasers for inertial confinement fusion or high average power drilling or burning applications.
Laser diodes find wide use in telecommunication as easily modulated and easily coupled light sources for fiber optics communication. They are used in various measuring instruments, such as rangefinders. Another common use is in barcode readers. Visible lasers, typically red but later also green, are common as laser pointers. Both low and high-power diodes are used extensively in the printing industry both as light sources for scanning (input) of images and for very high-speed and high-resolution printing plate (output) manufacturing. Infrared and red laser diodes are common in CD players, CD-ROMs and DVD technology. Violet lasers are used in HD DVD and Blu-ray technology. Diode lasers have also found many applications in laser absorption spectrometry (LAS) for high-speed, low-cost assessment or monitoring of the concentration of various species in gas phase. High-power laser diodes are used in industrial applications such as heat treating, cladding, seam welding and for pumping other lasers, such as diode pumped solid state lasers.
Applications of laser diodes can be categorized in various ways. Most applications could be served by larger solid state lasers or optical parametric oscillators, but the low cost of mass-produced diode lasers makes them essential for mass-market applications. Diode lasers can be used in a great many fields; since light has many different properties (power, wavelength and spectral quality, beam quality, polarization, etc.) it is interesting to classify applications by these basic properties.
Many applications of diode lasers primarily make use of the "directed energy" property of an optical beam. In this category one might include the laser printers, bar-code readers, image scanning, illuminators, designators, optical data recording, combustion ignition, laser surgery, industrial sorting, industrial machining, and directed energy weaponry. Some of these applications are emerging while others are well-established.
Laser medicine: medicine and especially dentistry have found many new applications for diode lasers.[6][7][8] The shrinking size of the units and their increasing user friendliness makes them very attractive to clinicians for minor soft tissue procedures. The 800 nm – 980 nm units have a high absorption rate for hemoglobin and thus make them ideal for soft tissue applications, where good hemostasis is necessary.
Applications which may make use of the coherence of diode-laser-generated light include interferometric distance measurement, holography, coherent communications, and coherent control of chemical reactions.
Applications which may make use of "narrow spectral" properties of diode lasers include range-finding, telecommunications, infra-red countermeasures, spectroscopic sensing, generation of radio-frequency or terahertz waves, atomic clock state preparation, quantum key cryptography, frequency doubling and conversion, water purification (in the UV), and photodynamic therapy (where a particular wavelength of light would cause a substance such as porphyrin to become chemically active as an anti-cancer agent only where the tissue is illuminated by light).
Applications where the desired quality of laser diodes is their ability to generate ultra-short pulses of light by the technique known as "mode-locking" include clock distribution for high-performance integrated circuits, high-peak-power sources for laser-induced breakdown spectroscopy sensing, arbitrary waveform generation for radio-frequency waves, photonic sampling for analog-to-digital conversion, and optical code-division-multiple-access systems for secure communication.
[edit] Common wavelengths
- 375 nm – excitation of Hoechst stain, Calcium Blue, and other fluorescent dyes in fluorescence microscopy
- 405 nm – InN blue-violet laser, in Blu-ray Disc and HD DVD drives
- 445 nm – InGaN Deep blue laser multimode diode recently introduced (2010) for use in mercury free high brightness data projectors
- 473 nm – Bright blue laser pointers, still very expensive, output of DPSS systems
- 485 nm – excitation of GFP and other fluorescent dyes
- 510 nm - Green diodes recently (2010) developed by Nichia for laser projectors.
- 532 nm – AlGaAs-pumped bright green laser pointers, frequency doubled 1064 nm Nd:YAG laser or (more commonly in laser pointers) Nd:YVO4 IR lasers (SHG)
- 593 nm – Yellow-Orange laser pointers, DPSS
- 635 nm – AlGaInP better red laser pointers, same power subjectively 5 times as bright as 670 nm one
- 640 nm –
- 650 nm – AlGaInP DVD drives, laser pointers
- 660 nm –
- 670 nm – AlGaInP cheap red laser pointers
- 785 nm – GaAlAs Compact Disc drives
- 808 nm – GaAlAs pumps in DPSS Nd:YAG lasers (e.g. in green laser pointers or as arrays in higher-powered lasers)
- 848 nm – laser mice
- 980 nm – InGaAs pump for optical amplifiers, for Yb:YAG DPSS lasers
- 1064 nm – AlGaAs fiber-optic communication
- 1310 nm – InGaAsP fiber-optic communication
- 1480 nm – InGaAsP pump for optical amplifiers
- 1550 nm – InGaAsP fiber-optic communication
- 1625 nm – InGaAsP fiber-optic communication, service channel
[edit] History
The first to demonstrate coherent light emission from a semiconductor diode (the first laser diode), is widely acknowledged to have been Robert N. Hall and his team at the General Electric research center in 1962.[9] The first visible wavelength laser diode was demonstrated by Nick Holonyak, Jr. later in 1962.[10]Other teams at IBM, MIT Lincoln Laboratory, Texas Instruments, and RCA Laboratories were also involved in and received credit for their historic initial demonstrations of efficient light emission and lasing in semiconductor diodes in 1962 and thereafter. GaAs lasers were also produced in early 1963 in the Soviet Union by the team led by Nikolay Basov.[11]
In the early 1960s liquid phase epitaxy (LPE) was invented by Herbert Nelson of RCA Laboratories. By layering the highest quality crystals of varying compositions, it enabled the demonstration of the highest quality heterojunction semiconductor laser materials for many years. LPE was adopted by all the leading laboratories, worldwide and used for many years. It was finally supplanted in the 1970s by molecular beam epitaxy and organometallic chemical vapor deposition.
Diode lasers of that era operated with threshold current densities of 1000 A/cm2 at 77 K temperatures. Such performance enabled continuous-lasing to be demonstrated in the earliest days. However, when operated at room temperature, about 300 K, threshold current densities were two orders of magnitude greater, or 100,000 A/cm2 in the best devices. The dominant challenge for the remainder of the 1960s was to obtain low threshold current density at 300 K and thereby to demonstrate continuous-wave lasing at room temperature from a diode laser.
The first diode lasers were homojunction diodes. That is, the material (and thus the bandgap) of the waveguide core layer and that of the surrounding clad layers, were identical. It was recognized that there was an opportunity, particularly afforded by the use of liquid phase epitaxy using aluminum gallium arsenide, to introduce heterojunctions. Heterostructures consist of layers of semiconductor crystal having varying bandgap and refractive index. Heterojunctions (formed from heterostructures) had been recognized by Herbert Kroemer, while working at RCA Laboratories in the mid-1950s, as having unique advantages for several types of electronic and optoelectronic devices including diode lasers. LPE afforded the technology of making heterojunction diode lasers.
The first heterojunction diode lasers were single-heterojunction lasers. These lasers utilized aluminum gallium arsenide p-type injectors situated over n-type gallium arsenide layers grown on the substrate by LPE. An admixture of aluminum replaced gallium in the semiconductor crystal and raised the bandgap of the p-type injector over that of the n-type layers beneath. It worked; the 300 K threshold currents went down by 10× to 10,000 amperes per square centimeter. Unfortunately, this was still not in the needed range and these single-heterostructure diode lasers did not function in continuous wave operation at room temperature.
The innovation that met the room temperature challenge was the double heterostructure laser. The trick was to quickly move the wafer in the LPE apparatus between different "melts" of aluminum gallium arsenide (p- and n-type) and a third melt of gallium arsenide. It had to be done rapidly since the gallium arsenide core region needed to be significantly under 1 µm in thickness. This may have been the earliest true example of "nanotechnology." The first laser diode to achieve continuous wave operation was a double heterostructure demonstrated in 1970 essentially simultaneously by Zhores Alferov and collaborators (including Dmitri Z. Garbuzov) of the Soviet Union, and Morton Panish and Izuo Hayashi working in the United States. However, it is widely accepted that Zhores I. Alferov and team reached the milestone first.
For their accomplishment and that of their co-workers, Alferov and Kroemer shared the 2000 Nobel Prize in Physics.
Quantum cascade laser
From Wikipedia, the free encyclopedia
Quantum cascade lasers (QCLs) are semiconductor lasers that emit in the mid- to far-infrared portion of the electromagnetic spectrum and were first demonstrated by Jerome Faist, Federico Capasso, Deborah Sivco, Carlo Sirtori, Albert Hutchinson, and Alfred Cho at Bell Laboratories in 1994.[1]Unlike typical interband semiconductor lasers that emit electromagnetic radiation through the recombination of electron–hole pairs across the material band gap, QCLs are unipolar and laser emission is achieved through the use of intersubband transitions in a repeated stack of semiconductor multiple quantum well heterostructures, an idea first proposed in the paper "Possibility of amplification of electromagnetic waves in a semiconductor with a superlattice" by R.F. Kazarinov and R.A. Suris in 1971.[2]
Contents[hide] |
[edit] Intersubband vs. interband transitions
Within a bulk semiconductor crystal, electrons may occupy states in one of two continuous energy bands - the valence band, which is heavily populated with low energy electrons and the conduction band, which is sparsely populated with high energy electrons. The two energy bands are separated by an energy band gap in which there are no permitted states available for electrons to occupy. Conventional semiconductor laser diodes generate light by a single photon being emitted when a high energy electron in the conduction band recombines with a hole in the valence band. The energy of the photon and hence the emission wavelength of laser diodes is therefore determined by the band gap of the material system used.A QCL however does not use bulk semiconductor materials in its optically active region. Instead it comprises a periodic series of thin layers of varying material composition forming a superlattice. The superlattice introduces a varying electric potential across the length of the device, meaning that there is a varying probability of electrons occupying different positions over the length of the device. This is referred to as one-dimensional multiple quantum well confinement and leads to the splitting of the band of permitted energies into a number of discrete electronic subbands. By suitable design of the layer thicknesses it is possible to engineer a population inversion between two subbands in the system which is required in order to achieve laser emission. Since the position of the energy levels in the system is primarily determined by the layer thicknesses and not the material, it is possible to tune the emission wavelength of QCLs over a wide range in the same material system.
[edit] Operating principles
[edit] Rate equations
 ,
,
 .
.
[edit] Active region designs
The scattering rates are tailored by suitable design of the layer thicknesses in the superlattice which determine the electron wave functions of the subbands. The scattering rate between two subbands is heavily dependent upon the overlap of the wave functions and energy spacing between the subbands. The figure shows the wave functions in a three quantum well (3QW) QCL active region and injector.In order to decrease W32, the overlap of the upper and lower laser levels is reduced. This is often achieved through designing the layer thicknesses such that the upper laser level is mostly localised in the left-hand well of the 3QW active region, while the lower laser level wave function is made to mostly reside in the central and right-hand wells. This is known as a diagonal transition. A vertical transition is one in which the upper laser level is localised in mainly the central and right-hand wells. This increases the overlap and hence W32 which reduces the population inversion, but it increases the strength of the radiative transition and therefore the gain.
In order to increase W21, the lower laser level and the ground level wave functions are designed such that they have a good overlap and to increase W21 further, the energy spacing between the subbands is designed such that it is equal to the longitudinal optical (LO) phonon energy (~36 meV in GaAs) so that resonant LO phonon-electron scattering can quickly depopulate the lower laser level.
[edit] Material systems
The first QCL was fabricated in the InGaAs/InAlAs material system lattice-matched to an InP substrate.[1] This particular material system has a conduction band offset (quantum well depth) of 520 meV.[citation needed] These InP-based devices have reached very high levels of performance across the mid-infrared spectral range, achieving high power, above room-temperature, continuous wave emission.[citation needed]In 1998 GaAs/AlGaAs QCLs were demonstrated by Sirtori et al. proving that the QC concept is not restricted to one material system.[citation needed] This material system has a varying quantum well depth depending on the aluminium fraction in the barriers.[citation needed] Although GaAs-based QCLs have not matched the performance levels of InP-based QCLs in the mid-infrared, they have proven to be very successful in the terahertz region of the spectrum.[citation needed]
The short wavelength limit of QCLs is determined by the depth of the quantum well and recently QCLs have been developed in material systems with very deep quantum wells in order to achieve short wavelength emission. The InGaAs/AlAsSb material system has quantum wells 1.6 eV deep and has been used to fabricate QCLs emitting at 3 μm.[citation needed] InAs/AlSb QCLs have quantum wells 2.1 eV deep and electroluminescence at wavelengths as short as 2.5 μm has been observed.[citation needed]
QCLs may also allow laser operation in materials traditionally considered to have poor optical properties. Indirect bandgap materials such as silicon have minimum electron and hole energies at different momentum values. For interband optical transitions, carriers change momentum through a slow, intermediate scattering process, dramatically reducing the optical emission intensity. Intersubband optical transitions however, are independent of the relative momentum of conduction band and valence band minima and theoretical proposals for Si/SiGe quantum cascade emitters have been made.[3]
[edit] Emission wavelengths
| This section requires expansion. |
[edit] Optical waveguides
Two types of optical waveguides are in common use. A ridge waveguide is created by etching parallel trenches in the quantum cascade gain material to create an isolated stripe of QC material, typically ~10 um wide, and several mm long. A dielectric material is typically deposited in the trenches to guide injected current into the ridge, then the entire ridge is typically coated with gold to provide electrical contact and to help remove heat from the ridge when it is producing light. Light is emitted from the cleaved ends of the waveguide, with an active area that is typically only a few micrometers in dimension.
The second waveguide type is a buried heterostructure. Here, the QC material is also etched to produce an isolated ridge. Now, however, new semiconductor material is grown over the ridge. The change in index of refraction between the QC material and the overgrown material is sufficient to create a waveguide. Dielectric material is also deposited on the overgrown material around QC ridge to guide the injected current into the QC gain medium. Buried heterostructure waveguides are efficient at removing heat from the QC active area when light is being produced.
[edit] Laser types
Although the quantum cascade gain medium can be used to produce incoherent light in a superluminescent configuration,[4] it is most commonly used in combination with an optical cavity to form a laser.[edit] Fabry–Pérot lasers
This is the simplest of the quantum cascade lasers. An optical waveguide is first fabricated out of the quantum cascade material to form the gain medium. The ends of the crystalline semiconductor device are then cleaved to form two parallel mirrors on either end of the waveguide, thus forming a Fabry–Pérot resonator. The residual reflectivity on the cleaved facets from the semiconductor-to-air interface is sufficient to create a resonator. Fabry–Pérot quantum cascade lasers are capable of producing high powers[5], but are typically multi-mode at higher operating currents. The wavelength can be changed chiefly by changing the temperature of the QC device.[edit] Distributed feedback lasers
A distributed feedback (DFB) quantum cascade laser[6] is similar to a Fabry–Pérot laser, except for a distributed Bragg reflector (DBR) built on top of the waveguide to prevent it from emitting at other than the desired wavelength. This forces single mode operation of the laser, even at higher operating currents. DFB lasers can be tuned chiefly by changing the temperature, although an interesting variant on tuning can be obtained by pulsing a DFB laser. In this mode, the wavelength of the laser is rapidly “chirped” during the course of the pulse, allowing rapid scanning of a spectral region. [7][edit] External cavity lasers
If a frequency-selective element is included in the external cavity, it is possible to reduce the laser emission to a single wavelength, and even tune the radiation. For example, diffraction gratings have been used to create[8] a tunable laser that can tune over 15% of its center wavelength.
[edit] Growth
| This section requires expansion. |
[edit] Applications
Distributed feedback (DFB) quantum cascade lasers were first commercialized in 2004,[9] and broadly-tunable external cavity quantum cascade lasers first commercialized in 2006.[10] The high optical power output, tuning range and room temperature operation make QCLs useful for spectroscopic applications such as remote sensing of environmental gases and pollutants in the atmosphere[11] and homeland security. They may eventually be used for vehicular cruise control in conditions of poor visibility,[citation needed] collision avoidance radar,[citation needed] industrial process control,[citation needed] and medical diagnostics such as breath analyzers.[citation needed] QCLs are also used to study plasma chemistry.[citation needed]Their large dynamic range,[clarification needed] excellent sensitivity,[clarification needed] and failsafe operation[clarification needed] combined with the solid-state reliability should easily[original research?] overcome many of the technological hurdles[clarification needed] that impede existing technology in these markets. When used in multiple-laser systems, intrapulse QCL spectroscopy[clarification needed] offers broadband spectral coverage that can potentially be used to identify and quantify complex heavy molecules such as those in toxic chemicals, explosives, and drugs.[12]
Unguided QCL emission in the 3–5 μm atmospheric window could be used as a cheaper alternative to optical fibres for high-speed Internet access in built up areas.[citation needed]
Light-emitting diode
From Wikipedia, the free encyclopedia
"LED" redirects here. For other uses, see LED (disambiguation).
| Light-emitting diode | |
|---|---|
 Red, green and blue LEDs of the 5mm type | |
| Type | Passive, optoelectronic |
| Working principle | Electroluminescence |
| Invented | Nick Holonyak Jr. (1962) |
| Electronic symbol | |
| Pin configuration | Anode and Cathode |
The LED is based on the semiconductor diode. When a diode is forward biased (switched on), electrons are able to recombine with holes within the device, releasing energy in the form of photons. This effect is called electroluminescence and the color of the light (corresponding to the energy of the photon) is determined by the energy gap of the semiconductor. An LED is usually small in area (less than 1 mm2), and integrated optical components are used to shape its radiation pattern and assist in reflection.[3] LEDs present many advantages over incandescent light sources including lower energy consumption, longer lifetime, improved robustness, smaller size, faster switching, and greater durability and reliability. LEDs powerful enough for room lighting are relatively expensive and require more precise current and heat management than compact fluorescent lamp sources of comparable output.
They are used in applications as diverse as replacements for aviation lighting, automotive lighting (particularly indicators) and in traffic signals. The compact size of LEDs has allowed new text and video displays and sensors to be developed, while their high switching rates are useful in advanced communications technology. Infrared LEDs are also used in the remote control units of many commercial products including televisions, DVD players, and other domestic appliances.
Contents[hide] |
[edit] History
[edit] Discoveries and early devices

Green electroluminescence from a point contact on a crystal of SiC recreates H. J. Round's original experiment from 1907.
In 1961, American experimenters Robert Biard and Gary Pittman working at Texas Instruments,[11] found that GaAs emitted infrared radiation when electric current was applied and received the patent for the infrared LED.
The first practical visible-spectrum (red) LED was developed in 1962 by Nick Holonyak Jr., while working at General Electric Company.[2] Holonyak is seen as the "father of the light-emitting diode".[12] M. George Craford,[13] a former graduate student of Holonyak, invented the first yellow LED and improved the brightness of red and red-orange LEDs by a factor of ten in 1972.[14] In 1976, T.P. Pearsall created the first high-brightness, high efficiency LEDs for optical fiber telecommunications by inventing new semiconductor materials specifically adapted to optical fiber transmission wavelengths.[15]
Up to 1968 visible and infrared LEDs were extremely costly, on the order of US $200 per unit, and so had little practical application.[16] The Monsanto Company was the first organization to mass-produce visible LEDs, using gallium arsenide phosphide in 1968 to produce red LEDs suitable for indicators.[16] Hewlett Packard (HP) introduced LEDs in 1968, initially using GaAsP supplied by Monsanto. The technology proved to have major applications for alphanumeric displays and was integrated into HP's early handheld calculators. In the 1970s commercially successful LED devices at under five cents each were produced by Fairchild Optoelectronics. These devices employed compound semiconductor chips fabricated with the planar process invented by Dr. Jean Hoerni at Fairchild Semiconductor.[17] The combination of planar processing for chip fabrication and innovative packaging techniques enabled the team at Fairchild led by optoelectronics pioneer Thomas Brandt to achieve the necessary cost reductions. These techniques continue to be used by LED producers.[18]
[edit] Practical use
The first commercial LEDs were commonly used as replacements for incandescent and neon indicator lamps, and in seven-segment displays,[19] first in expensive equipment such as laboratory and electronics test equipment, then later in such appliances as TVs, radios, telephones, calculators, and even watches (see list of signal applications). These red LEDs were bright enough only for use as indicators, as the light output was not enough to illuminate an area. Readouts in calculators were so small that plastic lenses were built over each digit to make them legible. Later, other colors became widely available and also appeared in appliances and equipment. As the LED materials technology became more advanced, the light output was increased, while maintaining the efficiency and the reliability to an acceptable level. The invention and development of the high power white light LED led to use for illumination[20][21] (see list of illumination applications). Most LEDs were made in the very common 5 mm T1¾ and 3 mm T1 packages, but with increasing power output, it has become increasingly necessary to shed excess heat in order to maintain reliability,[22] so more complex packages have been adapted for efficient heat dissipation. Packages for state-of-the-art high power LEDs bear little resemblance to early LEDs.
Illustration of Haitz's Law. Light output per LED as a function of production year, note the logarithmic scale on the vertical axis.
[edit] Continuing development
The first high-brightness blue LED was demonstrated by Shuji Nakamura of Nichia Corporation and was based on InGaN borrowing on critical developments in GaN nucleation on sapphire substrates and the demonstration of p-type doping of GaN which were developed by Isamu Akasaki and H. Amano in Nagoya. In 1995, Alberto Barbieri at the Cardiff University Laboratory (GB) investigated the efficiency and reliability of high-brightness LEDs and demonstrated a very impressive result by using a transparent contact made of indium tin oxide (ITO) on (AlGaInP/GaAs) LED. The existence of blue LEDs and high efficiency LEDs quickly led to the development of the first white LED, which employed a Y3Al5O12:Ce, or "YAG", phosphor coating to mix yellow (down-converted) light with blue to produce light that appears white. Nakamura was awarded the 2006 Millennium Technology Prize for his invention.[23]The development of LED technology has caused their efficiency and light output to increase exponentially, with a doubling occurring about every 36 months since the 1960s, in a way similar to Moore's law. The advances are generally attributed to the parallel development of other semiconductor technologies and advances in optics and material science. This trend is normally called Haitz's Law after Dr. Roland Haitz. [24]
In February 2008, Bilkent university in Turkey reported 300 lumens of visible light per watt luminous efficacy (not per electrical watt) and warm light by using nanocrystals.[25]
In January 2009, researchers from Cambridge University reported a process for growing gallium nitride (GaN) LEDs on silicon. Production costs could be reduced by 90% using six-inch silicon wafers instead of two-inch sapphire wafers. The team was led by Colin Humphreys.[26]
[edit] Technology
[edit] Physics
Like a normal diode, the LED consists of a chip of semiconducting material doped with impurities to create a p-n junction. As in other diodes, current flows easily from the p-side, or anode, to the n-side, or cathode, but not in the reverse direction. Charge-carriers—electrons and holes—flow into the junction from electrodes with different voltages. When an electron meets a hole, it falls into a lower energy level, and releases energy in the form of a photon.The wavelength of the light emitted, and therefore its color, depends on the band gap energy of the materials forming the p-n junction. In silicon or germanium diodes, the electrons and holes recombine by a non-radiative transition which produces no optical emission, because these are indirect band gap materials. The materials used for the LED have a direct band gap with energies corresponding to near-infrared, visible or near-ultraviolet light.
LED development began with infrared and red devices made with gallium arsenide. Advances in materials science have made possible the production of devices with ever-shorter wavelengths, producing light in a variety of colors.
LEDs are usually built on an n-type substrate, with an electrode attached to the p-type layer deposited on its surface. P-type substrates, while less common, occur as well. Many commercial LEDs, especially GaN/InGaN, also use sapphire substrate.
Most materials used for LED production have very high refractive indices. This means that much light will be reflected back into the material at the material/air surface interface. Therefore Light extraction in LEDs is an important aspect of LED production, subject to much research and development.
[edit] Efficiency and operational parameters
Typical indicator LEDs are designed to operate with no more than 30–60 milliwatts [mW] of electrical power. Around 1999, Philips Lumileds introduced power LEDs capable of continuous use at one watt [W]. These LEDs used much larger semiconductor die sizes to handle the large power inputs. Also, the semiconductor dies were mounted onto metal slugs to allow for heat removal from the LED die.One of the key advantages of LED-based lighting is its high efficiency, as measured by its light output per unit power input. White LEDs quickly matched and overtook the efficiency of standard incandescent lighting systems. In 2002, Lumileds made five-watt LEDs available with a luminous efficacy of 18–22 lumens per watt [lm/W]. For comparison, a conventional 60–100 W incandescent lightbulb produces around 15 lm/W, and standard fluorescent lights produce up to 100 lm/W. A recurring problem is that efficiency will fall dramatically for increased current. This effect is known as droop and effectively limits the light output of a given LED, increasing heating more than light output for increased current.[27][28][29]
In September 2003, a new type of blue LED was demonstrated by the company Cree, Inc. to provide 24 mW at 20 milliamperes [mA]. This produced a commercially packaged white light giving 65 lm/W at 20 mA, becoming the brightest white LED commercially available at the time, and more than four times as efficient as standard incandescents. In 2006 they demonstrated a prototype with a record white LED luminous efficacy of 131 lm/W at 20 mA. Also, Seoul Semiconductor has plans for 135 lm/W by 2007 and 145 lm/W by 2008, which would be approaching an order of magnitude improvement over standard incandescents and better even than standard fluorescents.[30] Nichia Corporation has developed a white LED with luminous efficacy of 150 lm/W at a forward current of 20 mA.[31]
High-power (≥ 1 W) LEDs are necessary for practical general lighting applications. Typical operating currents for these devices begin at 350 mA.
Note that these efficiencies are for the LED chip only, held at low temperature in a lab. In a lighting application, operating at higher temperature and with drive circuit losses, efficiencies are much lower. United States Department of Energy (DOE) testing of commercial LED lamps designed to replace incandescent lamps or CFLs showed that average efficacy was still about 46 lm/W in 2009 (tested performance ranged from 17 lm/W to 79 lm/W).[32]
Cree issued a press release on February 3, 2010 about a laboratory prototype LED achieving 208 lumens per watt at room temperature. The correlated color temperature was reported to be 4579 K.[33]
[edit] Lifetime and failure
Main article: List of LED failure modes
Solid state devices such as LEDs are subject to very limited wear and tear if operated at low currents and at low temperatures. Many of the LEDs produced in the 1970s and 1980s are still in service today. Typical lifetimes quoted are 25,000 to 100,000 hours but heat and current settings can extend or shorten this time significantly. [34]The most common symptom of LED (and diode laser) failure is the gradual lowering of light output and loss of efficiency. Sudden failures, although rare, can occur as well. Early red LEDs were notable for their short lifetime. With the development of high-power LEDs the devices are subjected to higher junction temperatures and higher current densities than traditional devices. This causes stress on the material and may cause early light output degradation. To quantitatively classify lifetime in a standardized manner it has been suggested to use the terms L75 and L50 which is the time it will take a given LED to reach 75% and 50% light output respectively.[35]
Like other lighting devices, LED performance is temperature dependent. Most manufacturers’ published ratings of LEDs are for an operating temperature of 25°C. LEDs used outdoors, such as traffic signals or in-pavement signal lights, and that are utilized in climates where the temperature within the luminaire gets very hot, could result in low signal intensities or even failure.[36]
LEDs maintain consistent light output even in cold temperatures, unlike traditional lighting methods. Consequently, LED technology may be a good replacement in areas such as supermarket freezer lighting[37][38][39] and will last longer than other technologies. Because LEDs do not generate as much heat as incandescent bulbs, they are an energy-efficient technology to use in such applications such as freezers. On the other hand, because they do not generate much heat, ice and snow may build up on the LED luminaire in colder climates.[40] This has been a problem plaguing airport runway lighting, although some research has been done to try to develop heat sink technologies in order to transfer heat to alternative areas of the luminaire.[41]
[edit] Colors and materials
Conventional LEDs are made from a variety of inorganic semiconductor materials, the following table shows the available colors with wavelength range, voltage drop and material:| Color | Wavelength (nm) | Voltage (V) | Semiconductor Material | |
|---|---|---|---|---|
| Infrared | λ > 760 | ΔV < 1.9 | Gallium arsenide (GaAs) Aluminium gallium arsenide (AlGaAs) | |
| Red | 610 < λ < 760 | 1.63 < ΔV < 2.03 | Aluminium gallium arsenide (AlGaAs) Gallium arsenide phosphide (GaAsP) Aluminium gallium indium phosphide (AlGaInP) Gallium(III) phosphide (GaP) | |
| Orange | 590 < λ < 610 | 2.03 < ΔV < 2.10 | Gallium arsenide phosphide (GaAsP) Aluminium gallium indium phosphide (AlGaInP) Gallium(III) phosphide (GaP) | |
| Yellow | 570 < λ < 590 | 2.10 < ΔV < 2.18 | Gallium arsenide phosphide (GaAsP) Aluminium gallium indium phosphide (AlGaInP) Gallium(III) phosphide (GaP) | |
| Green | 500 < λ < 570 | 1.9[42] < ΔV < 4.0 | Indium gallium nitride (InGaN) / Gallium(III) nitride (GaN) Gallium(III) phosphide (GaP) Aluminium gallium indium phosphide (AlGaInP) Aluminium gallium phosphide (AlGaP) | |
| Blue | 450 < λ < 500 | 2.48 < ΔV < 3.7 | Zinc selenide (ZnSe) Indium gallium nitride (InGaN) Silicon carbide (SiC) as substrate Silicon (Si) as substrate — (under development) | |
| Violet | 400 < λ < 450 | 2.76 < ΔV < 4.0 | Indium gallium nitride (InGaN) | |
| Purple | multiple types | 2.48 < ΔV < 3.7 | Dual blue/red LEDs, blue with red phosphor, or white with purple plastic | |
| Ultraviolet | λ < 400 | 3.1 < ΔV < 4.4 | Diamond (235 nm)[43] Boron nitride (215 nm)[44][45] Aluminium nitride (AlN) (210 nm)[46] Aluminium gallium nitride (AlGaN) Aluminium gallium indium nitride (AlGaInN) — (down to 210 nm)[47] | |
| White | Broad spectrum | ΔV = 3.5 | Blue/UV diode with yellow phosphor |
[edit] Ultraviolet and blue LEDs

Blue LEDs.
The first blue LEDs were made in 1971 by Jacques Pankove (inventor of the gallium nitride LED) at RCA Laboratories.[48] These devices had too little light output to be of much practical use. However, early blue LEDs found use in some low-light applications, such as the high-beam indicators for cars.[49] In the late 1980s, key breakthroughs in GaN epitaxial growth and p-type doping[50] ushered in the modern era of GaN-based optoelectronic devices. Building upon this foundation, in 1993 high brightness blue LEDs were demonstrated.[51]
By the late 1990s, blue LEDs had become widely available. They have an active region consisting of one or more InGaN quantum wells sandwiched between thicker layers of GaN, called cladding layers. By varying the relative InN-GaN fraction in the InGaN quantum wells, the light emission can be varied from violet to amber. AlGaN aluminium gallium nitride of varying AlN fraction can be used to manufacture the cladding and quantum well layers for ultraviolet LEDs, but these devices have not yet reached the level of efficiency and technological maturity of the InGaN-GaN blue/green devices. If the active quantum well layers are GaN, as opposed to alloyed InGaN or AlGaN, the device will emit near-ultraviolet light with wavelengths around 350–370 nm. Green LEDs manufactured from the InGaN-GaN system are far more efficient and brighter than green LEDs produced with non-nitride material systems.
With nitrides containing aluminium, most often AlGaN and AlGaInN, even shorter wavelengths are achievable. Ultraviolet LEDs in a range of wavelengths are becoming available on the market. Near-UV emitters at wavelengths around 375–395 nm are already cheap and often encountered, for example, as black light lamp replacements for inspection of anti-counterfeiting UV watermarks in some documents and paper currencies. Shorter wavelength diodes, while substantially more expensive, are commercially available for wavelengths down to 247 nm.[52] As the photosensitivity of microorganisms approximately matches the absorption spectrum of DNA, with a peak at about 260 nm, UV LED emitting at 250–270 nm are to be expected in prospective disinfection and sterilization devices. Recent research has shown that commercially available UVA LEDs (365 nm) are already effective disinfection and sterilization devices.[53]
Deep-UV wavelengths were obtained in laboratories using aluminium nitride (210 nm),[46] boron nitride (215 nm)[44][45] and diamond (235 nm).[43]
[edit] White light
There are two primary ways of producing high intensity white-light using LEDs. One is to use individual LEDs that emit three primary colors[54]—red, green, and blue—and then mix all the colors to produce white light. The other is to use a phosphor material to convert monochromatic light from a blue or UV LED to broad-spectrum white light, much in the same way a fluorescent light bulb works.Due to metamerism, it is possible to have quite different spectra that appear white.
[edit] RGB systems

Combined spectral curves for blue, yellow-green, and high brightness red solid-state semiconductor LEDs. FWHM spectral bandwidth is approximately 24–27 nm for all three colors.
There are several types of multi-colored white LEDs: di-, tri-, and tetrachromatic white LEDs. Several key factors that play among these different approaches include color stability, color rendering capability, and luminous efficacy. Often higher efficiency will mean lower color rendering, presenting a trade off between the luminous efficiency and color rendering. For example, the dichromatic white LEDs have the best luminous efficacy (120 lm/W), but the lowest color rendering capability. Conversely, although tetrachromatic white LEDs have excellent color rendering capability, they often have poor luminous efficiency. Trichromatic white LEDs are in between, having both good luminous efficacy (>70 lm/W) and fair color rendering capability.
What multi-color LEDs offer is not merely another solution of producing white light, but is a whole new technique of producing light of different colors. In principle, most perceivable colors can be produced by mixing different amounts of three primary colors, and this makes it possible to produce precise dynamic color control as well. As more effort is devoted to investigating this technique, multi-color LEDs should have profound influence on the fundamental method which we use to produce and control light color. However, before this type of LED can truly play a role on the market, several technical problems need to be solved. These certainly include that this type of LED's emission power decays exponentially with increasing temperature,[56] resulting in a substantial change in color stability. Such problems are not acceptable for industrial usage. Therefore, many new package designs aimed at solving this problem have been proposed and their results are now being reproduced by researchers and scientists.
[edit] Phosphor-based LEDs

Spectrum of a “white” LED clearly showing blue light which is directly emitted by the GaN-based LED (peak at about 465 nm) and the more broadband Stokes-shifted light emitted by the Ce3+:YAG phosphor which emits at roughly 500–700 nm.
Phosphor based LEDs have a lower efficiency than normal LEDs due to the heat loss from the Stokes shift and also other phosphor-related degradation issues. However, the phosphor method is still the most popular technique for manufacturing high intensity white LEDs. The design and production of a light source or light fixture using a monochrome emitter with phosphor conversion is simpler and cheaper than a complex RGB system, and the majority of high intensity white LEDs presently on the market are manufactured using phosphor light conversion.
The greatest barrier to high efficiency is the seemingly unavoidable Stokes energy loss. However, much effort is being spent on optimizing these devices to higher light output and higher operation temperatures. For instance, the efficiency can be increased by adapting better package design or by using a more suitable type of phosphor. Philips Lumileds' patented conformal coating process addresses the issue of varying phosphor thickness, giving the white LEDs a more homogeneous white light.[59] With development ongoing, the efficiency of phosphor based LEDs is generally increased with every new product announcement.
Technically the phosphor based white LEDs encapsulate InGaN blue LEDs inside of a phosphor coated epoxy. A common yellow phosphor material is cerium-doped yttrium aluminium garnet (Ce3+:YAG).
White LEDs can also be made by coating near ultraviolet (NUV) emitting LEDs with a mixture of high efficiency europium-based red and blue emitting phosphors plus green emitting copper and aluminium doped zinc sulfide (ZnS:Cu, Al). This is a method analogous to the way fluorescent lamps work. This method is less efficient than the blue LED with YAG:Ce phosphor, as the Stokes shift is larger and more energy is therefore converted to heat, but yields light with better spectral characteristics, which render color better. Due to the higher radiative output of the ultraviolet LEDs than of the blue ones, both approaches offer comparable brightness. Another concern is that UV light may leak from a malfunctioning light source and cause harm to human eyes or skin.
[edit] Other white LEDs
Another method used to produce experimental white light LEDs used no phosphors at all and was based on homoepitaxially grown zinc selenide (ZnSe) on a ZnSe substrate which simultaneously emitted blue light from its active region and yellow light from the substrate.[60][edit] Organic light-emitting diodes (OLEDs)
Main article: Organic light-emitting diode
If the emitting layer material of the LED is an organic compound, it is known as an organic light emitting diode (OLED). To function as a semiconductor, the organic emitting material must have conjugated pi bonds. [61] The emitting material can be a small organic molecule in a crystalline phase, or a polymer. Polymer materials can be flexible; such LEDs are known as PLEDs or FLEDs.Compared with regular LEDs, OLEDs are lighter, and polymer LEDs can have the added benefit of being flexible. Some possible future applications of OLEDs could be:
- Inexpensive, flexible displays
- Light sources
- Wall decorations
- Luminous cloth
Today, OLEDs operate at substantially lower efficiency than inorganic (crystalline) LEDs.[63]
[edit] Quantum dot LEDs (experimental)
A new technique developed by Michael Bowers, a graduate student at Vanderbilt University in Nashville, involves coating a blue LED with quantum dots that glow white in response to the blue light from the LED. This technique produces a warm, yellowish-white light similar to that produced by incandescent bulbs.[64]Quantum dots are semiconductor nanocrystals that possess unique optical properties.[65] Their emission color can be tuned from the visible throughout the infrared spectrum. This allows quantum dot LEDs to create almost any color on the CIE diagram. This provides more color options and better color rendering than white LEDs. Quantum dot LEDs are available in the same package types as traditional phosphor based LEDs.
In September 2009 Nanoco Group announced that it has signed a joint development agreement with a major Japanese electronics company under which it will design and develop quantum dots for use in light emitting diodes (LEDs) in liquid crystal display (LCD) televisions.[66]
[edit] Types
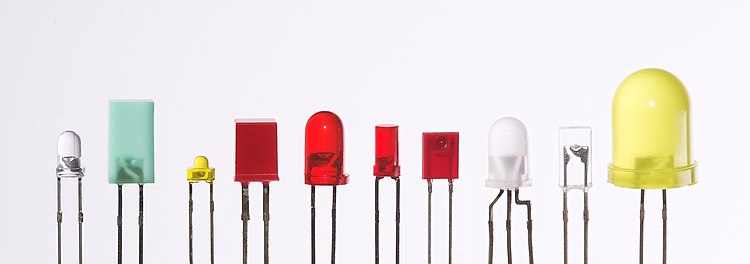
LEDs are produced in a variety of shapes and sizes. The 5 mm cylindrical package (red, fifth from the left) is the most common, estimated at 80% of world production.[citation needed] The color of the plastic lens is often the same as the actual color of light emitted, but not always. For instance, purple plastic is often used for infrared LEDs, and most blue devices have clear housings. There are also LEDs in SMT packages, such as those found on blinkies and on cell phone keypads (not shown).
[edit] Miniature LEDs
Main article: Miniature light-emitting diode
These are mostly single-die LEDs used as indicators, and they come in various-sizes from 2 mm to 8 mm, through-hole and surface mount packages. They are usually simple in design, not requiring any separate cooling body.[67] Typical current ratings ranges from around 1 mA to above 20 mA. The small scale sets a natural upper boundary on power consumption due to heat caused by the high current density and need for heat sinking.[edit] High power LEDs
See also: Solid-state lighting and LED lamp
High power LEDs (HPLED) can be driven at currents from hundreds of mA to more than an ampere, compared with the tens of mA for other LEDs. Some can produce over a thousand [68][69] lumens. Since overheating is destructive, the HPLEDs must be mounted on a heat sink to allow for heat dissipation. If the heat from a HPLED is not removed, the device will burn out in seconds. A single HPLED can often replace an incandescent bulb in a torch, or be set in an array to form a powerful LED lamp.Some well-known HPLEDs in this category are the Lumileds Rebel Led, Osram Opto Semiconductors Golden Dragon and Cree X-lamp. As of September 2009 some HPLEDs manufactured by Cree Inc. now exceed 105 lm/W [70] (e.g. the XLamp XP-G LED chip emitting Cool White light) and are being sold in lamps intended to replace incandescent, halogen, and even fluorescent style lights as LEDs become more cost competitive.
LEDs have been developed by Seoul Semiconductor that can operate on AC power without the need for a DC converter. For each half cycle part of the LED emits light and part is dark, and this is reversed during the next half cycle. The efficacy of this type of HPLED is typically 40 lm/W.[71] A large number of LED elements in series may be able to operate directly from line voltage. In 2009 Seoul Semiconductor released a high DC voltage capable of being driven from AC power with a simple controlling circuit. The low power dissipation of these LEDs affords them more flexibility than the original AC LED design.[citation needed]
[edit] Mid-range LEDs
Medium power LEDs are often through-hole mounted and used when a output of a few lumen is needed. They sometimes have the diode mounted to four leads (two cathode leads, two anode leads) for better heat conduction and carry an integrated lens. An example of this is the Superflux package, from Philips Lumileds. These LEDs are most commonly used in light panels, emergency lighting and automotive tail-lights. Due to the larger amount of metal in the LED, they are able to handle higher currents (around 100 mA). The higher current allows for the higher light output required for tail-lights and emergency lighting.[edit] Application-specific variations
- Flashing LEDs are used as attention seeking indicators without requiring external electronics. Flashing LEDs resemble standard LEDs but they contain an integrated multivibrator circuit which causes the LED to flash with a typical period of one second. In diffused lens LEDs this is visible as a small black dot. Most flashing LEDs emit light of a single color, but more sophisticated devices can flash between multiple colors and even fade through a color sequence using RGB color mixing.

Calculator LED display, 1970s.
- Bi-color LEDs are actually two different LEDs in one case. They consist of two dies connected to the same two leads antiparallel to each other. Current flow in one direction produces one color, and current in the opposite direction produces the other color. Alternating the two colors with sufficient frequency causes the appearance of a blended third color. For example, a red/green LED operated in this fashion will color blend to produce a yellow appearance.
- Tri-color LEDs are two LEDs in one case, but the two LEDs are connected to separate leads so that the two LEDs can be controlled independently and lit simultaneously. A three-lead arrangement is typical with one common lead (anode or cathode).[citation needed]
- RGB LEDs contain red, green and blue emitters, generally using a four-wire connection with one common lead (anode or cathode). These LEDs can have either common positive or common negative leads. Others however, have only two leads (positive and negative) and have a built in tiny electronic control unit.
- Alphanumeric LED displays are available in seven-segment and starburst format. Seven-segment displays handle all numbers and a limited set of letters. Starburst displays can display all letters. Seven-segment LED displays were in widespread use in the 1970s and 1980s, but increasing use of liquid crystal displays, with their lower power consumption and greater display flexibility, has reduced the popularity of numeric and alphanumeric LED displays.
[edit] Considerations for use
[edit] Power sources
Main article: LED power sources
The current/voltage characteristic of an LED is similar to other diodes, in that the current is dependent exponentially on the voltage (see Shockley diode equation). This means that a small change in voltage can lead to a large change in current. If the maximum voltage rating is exceeded by a small amount the current rating may be exceeded by a large amount, potentially damaging or destroying the LED. The typical solution is therefore to use constant current power supplies, or driving the LED at a voltage much below the maximum rating. Since most household power sources (batteries, mains) are not constant current sources, most LED fixtures must include a power converter. However, the I/V curve of nitride-based LEDs is quite steep above the knee and gives an If of a few milliamperes at a Vf of 3 V, making it possible to power a nitride-based LED from a 3 V battery such as a coin cell without the need for a current limiting resistor.[edit] Electrical polarity
Main article: Electrical polarity of LEDs
As with all diodes, current flows easily from p-type to n-type material.[72] However, no current flows and no light is produced if a small voltage is applied in the reverse direction. If the reverse voltage becomes large enough to exceed the breakdown voltage, a large current flows and the LED may be damaged. If the reverse current is sufficiently limited to avoid damage, the reverse-conducting LED is a useful noise diode.[edit] Safety
The vast majority of devices containing LEDs are "safe under all conditions of normal use", and so are classified as "Class 1 LED product"/"LED Klasse 1". At present, only a few LEDs—extremely bright LEDs that also have a tightly focused viewing angle of 8° or less—could, in theory, cause temporary blindness, and so are classified as "Class 2".[73] In general, laser safety regulations—and the "Class 1", "Class 2", etc. system—also apply to LEDs.[74][edit] Advantages
- Efficiency: LEDs produce more light per watt than incandescent bulbs.[75] Their efficiency is not affected by shape and size, unlike Fluorescent light bulbs or tubes.
- Color: LEDs can emit light of an intended color without the use of the color filters that traditional lighting methods require. This is more efficient and can lower initial costs.
- Size: LEDs can be very small (smaller than 2 mm2[76]) and are easily populated onto printed circuit boards.
- On/Off time: LEDs light up very quickly. A typical red indicator LED will achieve full brightness in under a microsecond.[77] LEDs used in communications devices can have even faster response times.
- Cycling: LEDs are ideal for use in applications that are subject to frequent on-off cycling, unlike fluorescent lamps that burn out more quickly when cycled frequently, or HID lamps that require a long time before restarting.
- Dimming: LEDs can very easily be dimmed either by pulse-width modulation or lowering the forward current.
- Cool light: In contrast to most light sources, LEDs radiate very little heat in the form of IR that can cause damage to sensitive objects or fabrics. Wasted energy is dispersed as heat through the base of the LED.
- Slow failure: LEDs mostly fail by dimming over time, rather than the abrupt burn-out of incandescent bulbs.[78]
- Lifetime: LEDs can have a relatively long useful life. One report estimates 35,000 to 50,000 hours of useful life, though time to complete failure may be longer.[79] Fluorescent tubes typically are rated at about 10,000 to 15,000 hours, depending partly on the conditions of use, and incandescent light bulbs at 1,000–2,000 hours.
- Shock resistance: LEDs, being solid state components, are difficult to damage with external shock, unlike fluorescent and incandescent bulbs which are fragile.
- Focus: The solid package of the LED can be designed to focus its light. Incandescent and fluorescent sources often require an external reflector to collect light and direct it in a usable manner.
- Toxicity: LEDs do not contain mercury, unlike fluorescent lamps.
[edit] Disadvantages
- Some Fluorescent lamps can be more efficient.
- High initial price: LEDs are currently more expensive, price per lumen, on an initial capital cost basis, than most conventional lighting technologies. The additional expense partially stems from the relatively low lumen output and the drive circuitry and power supplies needed.
- Temperature dependence: LED performance largely depends on the ambient temperature of the operating environment. Over-driving the LED in high ambient temperatures may result in overheating of the LED package, eventually leading to device failure. Adequate heat-sinking is required to maintain long life. This is especially important when considering automotive, medical, and military applications where the device must operate over a large range of temperatures, and is required to have a low failure rate.
- Voltage sensitivity: LEDs must be supplied with the voltage above the threshold and a current below the rating. This can involve series resistors or current-regulated power supplies.[80]
- Light quality: Most cool-white LEDs have spectra that differ significantly from a black body radiator like the sun or an incandescent light. The spike at 460 nm and dip at 500 nm can cause the color of objects to be perceived differently under cool-white LED illumination than sunlight or incandescent sources, due to metamerism,[81] red surfaces being rendered particularly badly by typical phosphor based cool-white LEDs. However, the color rendering properties of common fluorescent lamps are often inferior to what is now available in state-of-art white LEDs.
- Area light source: LEDs do not approximate a “point source” of light, but rather a lambertian distribution. So LEDs are difficult to use in applications requiring a spherical light field. LEDs are not capable of providing divergence below a few degrees. This is contrasted with lasers, which can produce beams with divergences of 0.2 degrees or less.[82]
- Blue hazard: There is a concern that blue LEDs and cool-white LEDs are now capable of exceeding safe limits of the so-called blue-light hazard as defined in eye safety specifications such as ANSI/IESNA RP-27.1-05: Recommended Practice for Photobiological Safety for Lamp and Lamp Systems.[83][84]
- Blue pollution: Because cool-white LEDs (i.e., LEDs with high color temperature) emit proportionally more blue light than conventional outdoor light sources such as high-pressure sodium lamps, the strong wavelength dependence of Rayleigh scattering means that cool-white LEDs can cause more light pollution than other light sources. The International Dark-Sky Association discourages the use of white light sources with correlated color temperature above 3,000 K.[citation needed]
[edit] Applications

LED lighting in a aircraft cabin of the Airbus A320 Enhanced.

A large LED display behind a disc jockey.

An information sign outside a parking garage in Sweden. The blinking effect is due to the rapid multiplexing of each LED row.

Traffic light using LED

Western Australia Police car using LED

Printhead of an Oki LED printer

LED daytime running lights of Audi A4

LED panel light source used in an experiment on plant growth. The findings of such experiments may be used to grow food in space on long duration missions.
- Visual signal application where the light goes more or less directly from the LED to the human eye, to convey a message or meaning.
- Illumination where LED light is reflected from object to give visual response of these objects.
- Generate light for measuring and interacting with processes that do not involve the human visual system.[85]
- Narrow band light sensors where the LED is operated in a reverse-bias mode and is responsive to incident light instead of emitting light.
[edit] Indicators and signs
The low energy consumption, low maintenance and small size of modern LEDs has led to applications as status indicators and displays on a variety of equipment and installations. Large area LED displays are used as stadium displays and as dynamic decorative displays. Thin, lightweight message displays are used at airports and railway stations, and as destination displays for trains, buses, trams, and ferries.The single color light is well suited for traffic lights and signals, exit signs, emergency vehicle lighting, ships' lanterns and LED-based Christmas lights. In cold climates, LED traffic lights may remain snow covered.[86] Red or yellow LEDs are used in indicator and alphanumeric displays in environments where night vision must be retained: aircraft cockpits, submarine and ship bridges, astronomy observatories, and in the field, e.g. night time animal watching and military field use.
Because of their long life and fast switching times, LEDs have been used for automotive high-mounted brake lights and truck and bus brake lights and turn signals for some time, but many vehicles now use LEDs for their rear light clusters. The use of LEDs also has styling advantages because LEDs are capable of forming much thinner lights than incandescent lamps with parabolic reflectors. The significant improvement in the time taken to light up (perhaps 0.5 s faster than an incandescent bulb) improves safety by giving drivers more time to react. It has been reported that at normal highway speeds this equals one car length increased reaction time for the car behind. White LED headlamps are beginning to make an appearance. In a dual intensity circuit(i.e. rear markers and brakes) if the LEDs are not pulsed at a fast enough frequency, they can create a phantom array, where ghost images of the LED will appear if the eyes quickly scan across the array.
Due to the relative cheapness of low output LEDs, they are also used in many temporary applications such as glowsticks, throwies, and the photonic textile Lumalive. Artists have also used LEDs for LED art.
Weather/all-hazards radio receivers with Specific Area Message Encoding (SAME) have three LEDs: red for warnings, orange for watches, and yellow for advisories & statements whenever issued.
[edit] Lighting
Main article: LED lamp
With the development of high efficiency and high power LEDs it has become possible to incorporate LEDs in lighting and illumination. Replacement light bulbs have been made as well as dedicated fixtures and LED lamps. LEDs are used as street lights and in other architectural lighting where color changing is used. The mechanical robustness and long lifetime is used in automotive lighting on cars, motorcycles and on bicycle lights.LED street lights are employed on poles and in parking garages. In 2007, the Italian village Torraca was the first place to convert its entire illumination system to LEDs.[87]
LEDs are used in aviation lighting. Airbus has used LED lighting in their Airbus A320 Enhanced since 2007, and Boeing plans its use in the 787. LEDs are also being used now in airport and heliport lighting. LED airport fixtures currently include medium intensity runway lights, runway centerline lights and obstruction lighting.
LEDs are also suitable for backlighting for LCD televisions and lightweight laptop displays and light source for DLP projectors (See LED TV). RGB LEDs increase the color gamut by as much as 45%. Screens for TV and computer displays can be made increasingly thin using LEDs for backlighting.[88]
LEDs are being used increasingly commonly for aquarium lighting. Particular for reef aquariums, LED lights provide an efficient light source with less heat output to help maintain optimal aquarium temperatures. LED-based aquarium fixtures also have the advantage of being manually adjustable to produce a specific color-spectrum for ideal coloration of corals, fish, and invertebrates while optimizing photosynethically active radiation (PAR) which increases growth and sustainability of photosynthetic life such as corals, anemones, clams, and macroalgae. These fixtures can be electronically programmed in order to simulate various lighting conditions throughout the day, reflecting phases of the sun and moon for a dynamic reef experience. LED fixtures typically cost up to five times as much as similarly rated fluorescent or high-intensity discharge lighting designed for reef aquariums and are not as high output to date.
The lack of IR/heat radiation makes LEDs ideal for stage lights using banks of RGB LEDs that can easily change color and decrease heating from traditional stage lighting, as well as medical lighting where IR-radiation can be harmful.
Since LEDs are small, durable and require little power they are used in hand held devices such as flashlights. LED strobe lights or camera flashes operate at a safe, low voltage, as opposed to the 250+ volts commonly found in xenon flashlamp-based lighting. This is particularly applicable to cameras on mobile phones, where space is at a premium and bulky voltage-increasing circuitry is undesirable. LEDs are used for infrared illumination in night vision applications including security cameras. A ring of LEDs around a video camera, aimed forward into a retroreflective background, allows chroma keying in video productions.
LEDs are used for decorative lighting as well. Uses include but are not limited to indoor/outdoor decor, limousines, cargo trailers, conversion vans, cruise ships, RVs, boats, automobiles, and utility trucks. Decorative LED lighting can also come in the form of lighted company signage and step and aisle lighting in theaters and auditoriums.
[edit] Smart lighting
Light can be used to transmit broadband data, which is already implemented in IrDA standards using infrared LEDs. Because LEDs can cycle on and off millions of times per second, they can, in effect, become wireless routers for data transport.[89] Lasers can also be modulated in this manner.[edit] Sustainable lighting
Efficient lighting is needed for sustainable architecture. A 13 watt LED lamp produces 450 to 650 lumens.[90] which is equivalent to a standard 40 watt incandescent bulb. [91] A standard 40 W incandescent bulb has an expected lifespan of 1,000 hours while an LED can continue to operate with reduced efficiency for more than 50,000 hours, 50 times longer than the incandescent bulb.[edit] Environmentally friendly options
A single kilowatt-hour of electricity will generate 1.34 pounds (610 g) of CO2 emissions.[92] Assuming the average light bulb is on for 10 hours a day, a single 40-watt incandescent bulb will generate 196 pounds (89 kg) of CO2 every year. The 13-watt LED equivalent will only be responsible for 63 pounds (29 kg) of CO2 over the same time span. A building’s carbon footprint from lighting can be reduced by 68% by exchanging all incandescent bulbs for new LEDs in warm climates. In cold climates, the energy saving may be lower, since more heating would be needed to compensate for the lower temperature.LEDs are also non-toxic unlike the more popular energy efficient bulb option: the compact fluorescent a.k.a. CFL which contains traces of harmful mercury. While the amount of mercury in a CFL is small, introducing less into the environment is preferable.
[edit] Economically sustainable
LED light bulbs could be a cost-effective option for lighting a home or office space because of their very long lifetimes. Consumer use of LEDs as a replacement for conventional lighting system is currently hampered by the high cost and low efficiency of available products. 2009 DOE testing results showed an average efficacy of 35 lm/W, below that of typical CFLs, and as low as 9 lm/W, worse than standard incandescents.[90] The high initial cost of the commercial LED bulb is due to the expensive sapphire substrate which is key to the production process. The sapphire apparatus must be coupled with a mirror-like collector to reflect light that would otherwise be wasted.In 2008, a materials science research team at Purdue University succeeded in producing LED bulbs with a substitute for the sapphire components.[93] The team used metal-coated silicon wafers with a built-in reflective layer of zirconium nitride to lessen the overall production cost of the LED. They predict that within a few years, LEDs produced with their revolutionary, new technique will be competitively priced with CFLs. The less expensive LED would not only be the best energy saver, but also a very economical bulb.
[edit] Non-visual applications
Light has many other uses besides for seeing. LEDs are used for some of these applications. The uses fall in three groups: Communication, sensors and light matter interaction.The light from LEDs can be modulated very quickly so they are used extensively in optical fiber and Free Space Optics communications. This include remote controls, such as for TVs and VCRs, where infrared LEDs are often used. Opto-isolators use an LED combined with a photodiode or phototransistor to provide a signal path with electrical isolation between two circuits. This is especially useful in medical equipment where the signals from a low voltage sensor circuit (usually battery powered) in contact with a living organism must be electrically isolated from any possible electrical failure in a recording or monitoring device operating at potentially dangerous voltages. An optoisolator also allows information to be transferred between circuits not sharing a common ground potential.
Many sensor systems rely on light as the signal source. LEDs are often ideal as a light source due to the requirements of the sensors. LEDs are used as movement sensors, for example in optical computer mice. The Nintendo Wii's sensor bar uses infrared LEDs. In pulse oximeters for measuring oxygen saturation. Some flatbed scanners use arrays of RGB LEDs rather than the typical cold-cathode fluorescent lamp as the light source. Having independent control of three illuminated colors allows the scanner to calibrate itself for more accurate color balance, and there is no need for warm-up. Furthermore, its sensors only need be monochromatic, since at any one point in time the page being scanned is only lit by a single color of light. Touch sensing: Since LEDs can also be used as photodiodes, they can be used for both photo emission and detection. This could be used in for example a touch-sensing screen that register reflected light from a finger or stylus.[94]
Many materials and biological systems are sensitive to, or dependent on light. Grow lights use LEDs to increase photosynthesis in plants[95] and bacteria and viruses can be removed from water and other substances using UV LEDs for sterilization.[53] Other uses are as UV curing devices for some ink and coating applications as well as LED printers.
The use of LEDs is particularly interesting to plant cultivators, mainly because it is more energy efficient, less heat is produced (can damage plants close to hot lamps) and can provide the optimum light frequency for plant growth and bloom periods compared to currently used grow lights: HPS (high pressure sodium), MH (metal halide) or CFL/low-energy. It has however not replaced these grow lights due to it having a higher retail price, as mass production and LED kits develop the product will become cheaper.
LEDs have also been used as a medium quality voltage reference in electronic circuits. The forward voltage drop (e.g., about 1.7 V for a normal red LED) can be used instead of a Zener diode in low-voltage regulators. Red LEDs have the flattest I/V curve above the knee; nitride-based LEDs have a fairly steep I/V curve and are not useful in this application. Although LED forward voltage is much more current-dependent than a good Zener, Zener diodes are not widely available below voltages of about 3 V.
[edit] Light sources for machine vision systems
Machine vision systems often require bright and homogeneous illumination, so features of interest are easier to process. LEDs are often used to this purpose, and this field of application is likely to remain one of the major application areas until price drops low enough to make signaling and illumination applications more widespread. Barcode scanners are the most common example of machine vision, and many inexpensive ones used red LEDs instead of lasers. LEDs constitute a nearly ideal light source for machine vision systems for several reasons:The size of the illuminated field is usually comparatively small and machine vision systems are often quite expensive, so the cost of the light source is usually a minor concern. However, it might not be easy to replace a broken light source placed within complex machinery, and here the long service life of LEDs is a benefit.
LED elements tend to be small and can be placed with high density over flat or even shaped substrates (PCBs etc.) so that bright and homogeneous sources can be designed which direct light from tightly controlled directions on inspected parts. This can often be obtained with small, inexpensive lenses and diffusers, helping to achieve high light densities with control over lighting levels and homogeneity. LED sources can be shaped in several configurations (spot lights for reflective illumination; ring lights for coaxial illumination; back lights for contour illumination; linear assemblies; flat, large format panels; dome sources for diffused, omnidirectional illumination).
LEDs can be easily strobed (in the microsecond range and below) and synchronized with imaging. High power LEDs are available allowing well lit images even with very short light pulses. This is often used in order to obtain crisp and sharp “still” images of quickly moving parts.
LEDs come in several different colors and wavelengths, easily allowing to use the best color for each application, where different color may provide better visibility of features of interest. Having a precisely known spectrum allows tightly matched filters to be used to separate informative bandwidth or to reduce disturbing effect of ambient light. LEDs usually operate at comparatively low working temperatures, simplifying heat management and dissipation, therefore allowing plastic lenses, filters and diffusers to be used. Waterproof units can also easily be designed, allowing for use in harsh or wet environments (food, beverage, oil industries).




















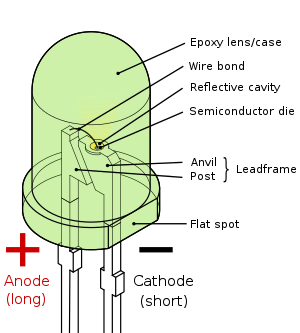

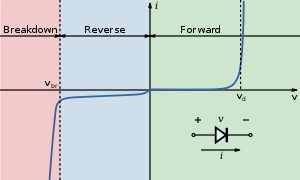




No comments:
Post a Comment